Abstract
Introduction: Large randomized trials investigating targeted agents have resulted in a markedly improved progression-free survival (PFS) when compared to standard chemoimmunotherapy. However, for small subgroups with a favorable risk profile, overall survival was not consistently superior for targeted agents. Long-term follow up on FCR suggested that favorable risk patients (pts) benefit significantly from this therapy and can expect an exceptionally long PFS. In addition, targeted first line (1L) therapies for favorable risk pts are reimbursed only in a minority of countries. Here, we report long-term efficacy data of FCR treatment obtained from pts documented within the GCLLSG registry.
Methods: Documentation within the GCLLSG registry started in 2013 and included both pts from prospective trials (29.7%), most importantly of the CLL8 trial (22.6%) and CLL10 trial (62.1%) as well as retrospective data. Pts with confirmed diagnosis of CLL who received FCR as 1L treatment were included. Patient characteristics were reported in median, interquartile ranges or frequencies and percentages. Response was reported as response or non-response. Event-free survival (EFS), which was defined as the time from start of (first) FCR treatment to disease progression, initiation of subsequent treatment for CLL or death. EFS was chosen as an endpoint for this report due to concerns that PFS might give unrealistically good results due to lack of documentation. However, PFS and TTNT data will be presented at the meeting.
Overall survival was calculated from initiation of 1L treatment for all pts who received 1L treatment after 2013. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated by Cox proportional hazards regression. Variables that were significantly associated with EFS in univariate analyses (significance level was set at 5%) were considered as candidates for the multivariate modelling to identify independent prognostic risk factors.
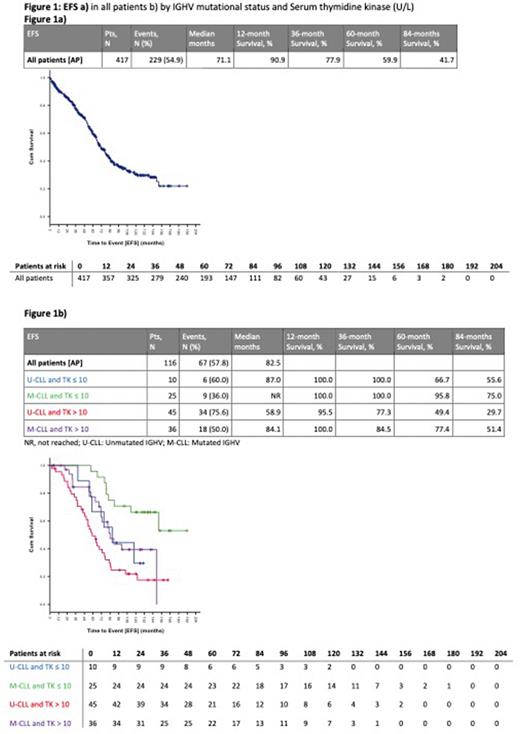
Results: As of September 2021, a total of 5922 pts were enrolled in the registry of which 417 received FCR as 1L treatment between 2003 and 2021. 223 pts (53.5%) received treatment before 2013 and 194 (46.5%) after 2013. Median observation time from 1L treatment was 95.8 (interquartile range 58.7-126.8) months. Patient characteristics showed a median age of 58 (range 24-83) years. Unmutated IGHV (U-CLL) was present in 61.4% of the pts. Regarding genomic aberrations based on the hierarchical model, del(17p) was found in 1.9%, del(11q) in 15.2%, trisomy12 in 15.5%, mono-allelic del(13q) in 34.3% and none of these changes in 33.0% of the pts. 88.8% of the pts responded to first line therapy, 11.2% were documented as non-responder. Median EFS was 71.1 month, for 188 pts no event was documented at the time point of data cut-off. Age group (≤ 65 years, >65 years), del(11q), IGHV mutational status, TP53 status, and serum thymidine kinase (TK) were associated with EFS in univariate analyses. Overall, 116 pts with 67 events were included in the final multivariable model for EFS. U-CLL (HR 1.949 (95%CI: 1.170-3.247; p=0.010) and TK >10 U/l (HR 2.107 (95%CI: 1.153-3.849; p=0.015) were independent adverse prognostic risk factors for EFS. When low TK and M-CLL were combined, the median EFS was not reached, with a 5-year survival rate of 95.8%. In contrast, median EFS was 84.1 months for pts with U-CLL and high TK. In U-CLL pts, median EFS was 87.0 months for pts with TK ≤ 10 U/l, and 58.9 months for pts with TK > 10 U/l. Median overall survival was not reached with a 5-year survival rate of 92.7%.
Conclusion: Within this cohort of pts with a long follow up in the GCLLSG registry, FCR treatment was associated with a long EFS especially in pts with a low risk profile exhibiting a mutated IGHV status und low TK.
Disclosures
Kutsch:Astra Zeneca: Honoraria, Other: Travel support; Janssen: Other: Travel Support; Celgene: Other: Travel Support; Gilead Sciences, Inc.: Other: Travel Support, Research Funding; Roche: Honoraria. Fink:Astra Zeneca: Research Funding; Celgene: Research Funding; AbbVie: Other: Travel Support. Robrecht:AstraZeneca: Honoraria. Koenigsmann:Novartis: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria; AbbVie: Honoraria; Janssen: Honoraria; Roche: Honoraria; Celgene: Honoraria. Tausch:Abbvie: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Honoraria, Speakers Bureau; Janssen-Cilag: Honoraria; Roche: Consultancy, Research Funding, Speakers Bureau; BeiGene: Honoraria. Schneider:AbbVie, AstraZeneca: Honoraria, Speakers Bureau. Stilgenbauer:Veristem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sunesis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Infinity: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Hoffmann-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Acerta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AbbVIe: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Beigene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Fischer:AbbVie: Honoraria; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support. Eichhorst:Janssen, Roche, AbbVie, BeiGene, AstraZeneca: Research Funding; Janssen, AbbVie, Lilly, AstraZeneca, BeiGene, MSD: Consultancy; Beigene: Other: Travel Support; Janssen, Roche, AbbVie, BeiGene, AstraZeneca, MSD: Speakers Bureau. Hallek:Abbvie, AstraZeneca: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria; Roche, Janssen: Honoraria, Research Funding; Gilead: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal